A biocatalytic redox cascade approach for one-pot deracemization of carboxyl-substituted tetrahydroisoquinolines by stereoinversion†
Abstract
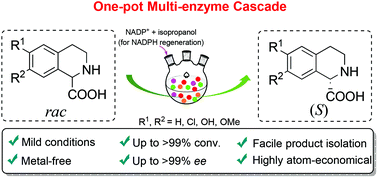
Optically pure 1,2,3,4-tetrahydroisoquinoline carboxylic acids are important chiral building blocks in the pharmaceutical and fine chemical industries. However, the existing chemo-enzymatic deracemization method employing D-amino acid oxidase from Fusarium solani M-0718 (FsDAAO) suffers from the requirement for a large excess of a nonselective chemical reducing agent. To explore an alternative method, we envisaged a concurrent biocatalytic oxidation and reduction cascade in one pot. Herein, we report a novel biocatalytic route for the asymmetric reduction of 3,4-dihydroisoquinoline-1-carboxylic acids employing Δ1-piperidine-2-carboxylate/Δ1-pyrrolidine-2-carboxylate reductase from Pseudomonas putida KT2440 (PpDpkA) as a biocatalyst, yielding the corresponding (S)-1-carboxyl-substituted tetrahydroisoquinolines with high conversions and enantiomeric excess (>99% ee). By combining FsDAAO and PpDpkA in one pot, a fully biocatalytic method was demonstrated for the deracemization of a range of racemic 1-carboxyl substituted tetrahydroisoquinolines to produce the corresponding (S)-enantiomers with >99% conversions and >99% ee. Furthermore, preparative-scale biotransformation of racemic 1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid gave the (S)-enantiomer with 89% isolated yield and >99% ee. Taken together, we provide an enantioselective biocatalytic redox cascade method for the one-pot synthesis of enantiopure 1,2,3,4-tetrahydroisoquinoline carboxylic acids.


 Please wait while we load your content...
Please wait while we load your content...