Selective hydrothermal reductions using geomimicry†
Abstract
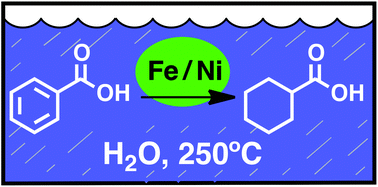
Reduction of carbon–carbon π-bonds has been demonstrated using iron powder as the reductant and simple powdered nickel as the catalyst in water as the solvent at 250 °C and the saturated water vapor pressure, 40 bars. Stereochemical, kinetic and electronic probes of the mechanism suggest reaction via a conventional Horiuti–Polyani process for hydrogenation at the nickel metal surface. Selective reduction of carbon–carbon π-bonds is observed in the presence of other functional groups. The reactions use benign and Earth-abundant reagents that are at low depletion risk and take place in water as the only solvent under conditions that are characteristic of many geochemical processes.


 Please wait while we load your content...
Please wait while we load your content...