Ru-Catalyzed methanol homologation with CO2 and H2 in an ionic liquid†
Abstract
Synthesis of ethanol from cheap and renewable CO2 is of great importance, but is still a challenge. Because it involves CO2 hydrogenation with simultaneous C–C bond formation, and the synergy of two or more transition metals is usually required for catalyzing the reaction effectively. Moreover, higher temperature is needed to drive the reaction. In this work, we found that the monometallic Ru3(CO)12 catalyst could efficiently catalyze the synthesis of ethanol from CO2, methanol and H2 in the ionic liquid, 1-butyl-3-methylimidazolium chloride ([bmim]Cl), using LiCl and LiI as promoters. Ethanol could form at 120 °C, which is among the lowest temperatures reported to date. A detailed study indicated that ionic liquids play an important role in monometallic catalysis. High ethanol selectivity and activity were obtained under optimized conditions. In addition, the catalyst could be easily recycled and the TON of ethanol reached 180 after five cycles. To our knowledge, this is the first time that an ionic liquid has been used as a reaction medium to produce ethanol using CO2 and H2.
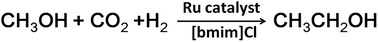


 Please wait while we load your content...
Please wait while we load your content...