CO2 adsorption on hydroxylated In2O3(110)†
Abstract
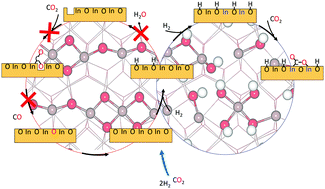
Catalytic synthesis of methanol from CO2 is one route to produce added-value chemicals from a greenhouse gas. Here, density functional theory calculations and ab initio thermodynamics are used to study CO2 adsorption on In2O3(110) in the presence of H2 and H2O. We find that the surface is heavily hydroxylated by either H2 or H2O and that hydroxylation promotes H2-induced vacancy formation. Moreover, CO2 adsorbs rather in a CO2− configuration on hydroxylated In2O3(110) than on oxygen vacancy sites. The results suggest that hydroxylation-induced oxidation-state changes of In-ions play a significant role in CO2 adsorption and activation during methanol synthesis.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...