Theoretical study on the gas phase reaction of CH2O + NH3: the formation of CH2O⋯NH3, NH2CH2OH, or CH2NH + H2O†
Abstract
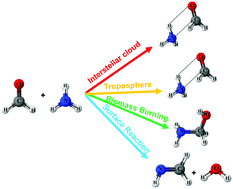
The gas phase reaction between CH2O and NH3 is an important reaction in cold interstellar clouds, combustion chemistry and organic chemistry. In this study, the stationary point on the potential energy surfaces (PESs) for the CH2O + NH3 reaction was computed at the CCSD(T)/6-311++G(3df,3pd)//M06-2X/6-311++G(3df,3pd) level. The temperature- and pressure-dependent rate constants were computed using advanced kinetic models, including microcanonical variational transition state theory and Rice–Ramsperger–Kassel–Marcus (RRKM)/master equation (ME) techniques. Our result predicts that the CH2O + NH3 reaction forms a collisionally thermalized CH2O⋯NH3 complex with respect to thermal unimolecular dissociation and the other products, i.e., NH2CH2OH and CH2NH + H2O, are negligible under atmospheric conditions. The calculated atmospheric lifetime of the CH2O⋯NH3 complex is ∼17 min, which suggests that the CH2O⋯NH3 complex can react with other atmospheric species. The results also suggest that the formation of CH2NH and H2O from the Strecker's process is negligibly small under all the conditions studied here. The decay rate of CH2O + NH3 (5.1 × 10−4 s−1 at 1500 K) suggests that aminomethanol (NH2CH2OH) is likely to occur in the high-temperature combustion of biomass burning, but the rate of formation of NH2CH2OH is negligible under atmospheric conditions. The predicted atmospheric lifetime (∼4 days) of NH2CH2OH in the presence of the OH radical suggests that further reactions with other atmospheric species are possible. The formation of the NH2CHOH radical from the reaction OH + NH2CH2OH can lead to carcinogenic products, such as nitrosamines, acetamide, hydrocycnic acid, NH2 and CO2.


 Please wait while we load your content...
Please wait while we load your content...