Structural inhomogeneity as a factor promoting the homogenous catalysis of CO2 hydrogenation by (PMe3)4RuH2†
Abstract
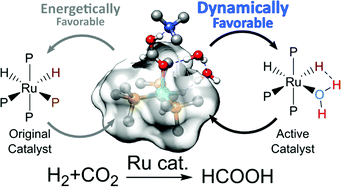
During homogenous catalysis by organometallic complexes, the dissociation of a ligand to produce an unsaturated site on the metal center is often invoked as the first step of activation, especially when photo-excitation is involved. In this theoretical study, we demonstrated that under mild conditions, a thermodynamically unstable yet dynamically favorable active intermediate could be produced by the inhomogeneity of the solvent distribution around the catalyst rather than by ligand dissociation. This occurred at the end of the first catalytic cycle when the product was eliminated. The empty site was immediately filled by one of the additive molecules aggregated around the reaction center even when the intermediate complex was unstable, producing a transient and more active catalyst. This process accounted for the accelerated reaction rate observed in the landmark CO2 hydrogenation catalyzed by (PMe3)4RuH2 in supercritical CO2 when H2O, MeOH, or HNMe2 was added. This also suggests a new way to exploit the structural inhomogeneity around an organometallic complex for the design of superior catalysts.


 Please wait while we load your content...
Please wait while we load your content...