Temperature dependent specific ion effects in mixed salt environments on a thermoresponsive poly(oligoethylene glycol methacrylate) brush†
Abstract
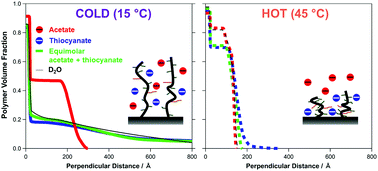
The temperature induced swelling/collapse transition of poly(oligoethylene glycol methacrylate) (POEGMA) brushes has been investigated in electrolyte solutions comprised of multiple anions. The behaviour of a POEGMA brush in mixed salt environments of potassium acetate (KCH3COO, causes collapse) and thiocyanate (KSCN, causes swelling), two ions at opposite ends of the Hofmeister series, has been monitored with neutron reflectometry (NR) and quartz crystal microbalance with dissipation (QCM-D). These techniques revealed that the balance of the swelling/collapse influence of the two ions on the structure of the brush is temperature dependent. At low temperatures in mixed salt environments, the influence of the acetate and thiocyanate ions appears additive, antagonistic and approximately equal in magnitude, with brush thickness and dissipation similar to the brush in the absence of electrolyte. At higher temperatures, the influence of the acetate ion diminishes, resulting in an increase in the relative influence of the thiocyanate ion on the brush conformation. These temperature dependent specific ion effects are attributed to increased steric crowding in the brush, along with an increased affinity of the thiocyanate ion for the polymer at higher temperatures.


 Please wait while we load your content...
Please wait while we load your content...