Naphthalene crystal shape prediction from molecular dynamics simulations†
Abstract
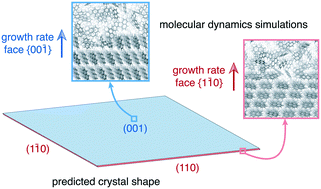
We used molecular dynamics simulations to predict the steady state crystal shape of naphthalene grown from ethanol solution. The simulations were performed at constant supersaturation by utilizing a recently proposed algorithm [Perego et al., J. Chem. Phys., 2015, 142, 144113]. To bring the crystal growth within the timescale of a molecular dynamics simulation we applied well-tempered metadynamics with a spatially constrained collective variable, which focuses the sampling on the growing layer. We estimated that the resulting steady state crystal shape corresponds to a rhombic prism, which is in line with experiments. Further, we observed that at the investigated supersaturations, the {00![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) } face grows in a two step two dimensional nucleation mechanism while the considerably faster growing faces {1
} face grows in a two step two dimensional nucleation mechanism while the considerably faster growing faces {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0} and {20
0} and {20![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) } grow new layers with a one step two dimensional nucleation mechanism.
} grow new layers with a one step two dimensional nucleation mechanism.
- This article is part of the themed collection: Editor’s Collection: Computer aided solid form design


 Please wait while we load your content...
Please wait while we load your content...