A Zn(ii) metal–organic framework constructed by a mixed-ligand strategy for CO2 capture and gas separation†
Abstract
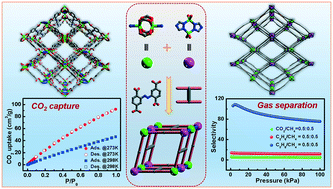
By introducing the 1,2,4-triazole ligand in the tetracarboxylate system, an anionic metal–organic framework [(CH3)2NH2][Zn2(ABTC)(Tz)]·3DMF (compound 1, ABTC = 2,2′,5,5′-azobenzene tetracarboxylic acid, Tz = 1,2,4-triazole and DMF = N,N-dimethylformamide) was synthesized under solvothermal conditions. There were two types of inorganic secondary building units (SBUs) in compound 1, i.e., a classical Zn-paddlewheel [Zn2(COO)4] and a metal cluster of [Zn2(Tz)2(COO)4], which were linked by ABTC4− to form a 3D porous framework. Because of the two inorganic SBUs as 6-connected nodes and ABTC4− as 4-connected nodes, compound 1 could be simplified to with a point symbol of (32·62·72) (34·42·64·75). Compound 1 displayed CO2 adsorption capacity of 92.1 cm3 g−1 at 273 K under 1 atm. Furthermore, 1 exhibited good adsorption capacity for small molecular gases (CH4, C2H6 and C3H8) and excellent separation selectivity for CO2/CH4, C2H6/CH4 and C3H8/CH4.


 Please wait while we load your content...
Please wait while we load your content...