Enantioselective iridium catalyzed α-alkylation of azlactones by a tandem asymmetric allylic alkylation/aza-Cope rearrangement†
Abstract
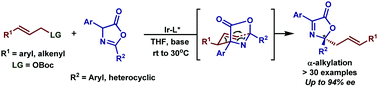
The development of an iridium catalyzed enantioselective α-alkylation of azlactones has been described. The reaction provides rapid access to a wide range of enantio-enriched quaternary carbon center allylated 2,4-diaryloxazol-5(2H)-ones in excellent yields with high enantioselectivities. The transformation was achieved through a tandem allylic alkylation/aza-Cope rearrangement, affording the desired products under mild conditions. Ultimately, the resulting products could be readily converted into diverse enantio-enriched derivatives which highlight the importance of the methodology.


 Please wait while we load your content...
Please wait while we load your content...