Ligand assisted electrocatalytic water oxidation by a copper(ii) complex in neutral phosphate buffer†
Abstract
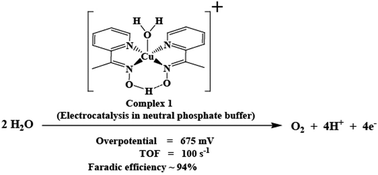
The electrocatalytic water oxidation activity of a copper(II) complex, 1, [Cu(L1H)(L1)(OH2)](ClO4), with a redox active aryl oxime ligand, L1H [L1H = 1-(pyridin-2-yl) ethanone oxime] has been investigated. Complex 1 shows a remarkably high turnover frequency of ∼100 s−1 in neutral phosphate buffer at about 675 mV overpotential with ∼94% faradaic efficiency. Electrochemical analysis suggests the involvement of a ligand moiety in a proton-coupled-electron-transfer (PCET) step during the catalytic cycle of complex 1, which in turn provides a route for accumulation of high oxidizing equivalents at the reaction center.


 Please wait while we load your content...
Please wait while we load your content...