Ruthenium-catalyzed ortho-selective CAr–H amination of heteroaryl arenes with di-tert-butyldiaziridinone†
Abstract
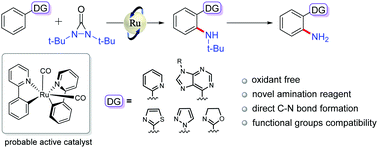
Application of an oxidative amination reagent (di-tert-butyldiaziridinone) to the Ru3(CO)12-catalyzed ortho-selective CAr–H amination reaction is described. This strategy shows good functional group compatibility with various phenyl-substituted N-heterocycles, including biologically active substrates, thus providing synthetic potential for this methodology. Mechanistic studies showed that the reaction process involves an octahedral ruthenium species, and the carbon monoxide ligand plays a crucial role in the C–H activation.


 Please wait while we load your content...
Please wait while we load your content...