Dual-responsive BN-embedded phenacenes featuring mechanochromic luminescence and ratiometric sensing of fluoride ions†
Abstract
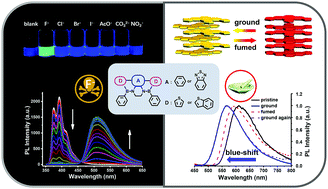
A series of novel dual-responsive BN-embedded phenacenes were designed and synthesized, via N-directed borylation of donor–acceptor–donor (D–A–D) precursors as the key step. Their crystal structures, and photophysical and electrical properties were systematically studied, which were found to be highly dependent on the incorporated BN units and fused aromatic rings with varied electron density. For the first time, mechanochromic luminescent (MCL) properties of BN-embedded phenacenes were realized, which are regulated jointly by inter- and intra-molecular effects at the molecular level. In the solid state, their emission colors can be tuned reversibly by external force, and interestingly, they exhibit an unusual hypochromic shift after grinding. Furthermore, in solution, these BN-containing phenacenes enable colorimetric and fluorometric detection of fluoride ions with high selectivity and a low detection limit of down to 1.8 × 10−8 M. The dual-responsive properties of these compounds highlight the widespread applications of BN containing conjugated materials in optical sensors.


 Please wait while we load your content...
Please wait while we load your content...