Effect of donor units in methylated DPP-based polymers on performance of organic field-effect transistors†
Abstract
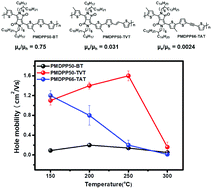
A series of new donor–acceptor copolymers containing methylated diketopyrrolopyrrole (MDPP) was synthesized for the active layer of organic field-effect transistors (OFETs) through Stille cross-coupling with various donor units including bithiophene (PMDPP50-BT), thienylene–vinylene–thienylene (PMDPP50-TVT), and thienylene–acetylene–thienylene (PMDPP66-TAT). The performance of the OFETs strongly depended on which donor unit was copolymerized with MDPP. The PMDPP50-BT OFETs exhibited a well-balanced hole and electron mobility of 0.2 ± 0.04 and 0.15 ± 0.04 cm2 V−1 s−1, respectively, even though their absolute mobility values were the lowest due to their high crystalline randomness and poor conjugated backbone planarity. By changing the donor unit from BT to the larger-sized TVT or TAT, both the PMDPP50-TVT and PMDPP66-TAT OFETs showed significantly improved hole mobilities of 1.6 ± 0.1 and 1.25 ± 0.1 cm2 V−1 s−1, respectively, due to their more extended backbone planarity and well-developed edge-on orientation. However, the electron mobilities of the PMDPP50-TVT and PMDPP66-TAT OFETs degraded to 0.05 and 0.003 cm2 V−1 s−1, respectively, because of their relatively high lowest unoccupied molecular orbital energy levels resulting in poor electron injection properties.


 Please wait while we load your content...
Please wait while we load your content...