A rechargeable metal-free full-liquid sulfur–bromine battery for sustainable energy storage†
Abstract
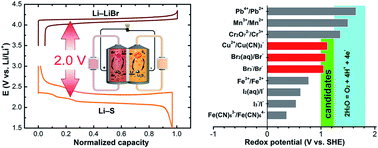
The broad application of lithium–sulfur technology is far from viable unless the obstacles associated with the dissolution of the sulfur cathode and the dendrite-growth related battery failure arising from the use of a metallic lithium anode are addressed. Taking advantage of the highly soluble sulfur species, this work explores the possibility of using redox-active species with highly positive potential to couple with a sulfur anolyte for a redox flow battery. When paired with an aqueous bromide catholyte, a sulfur–bromine (S–Br2) battery with the desired metal-free characteristic is successfully demonstrated. The battery exhibits a cell voltage exceeding 1.8 V, a specific capacity of ∼1600 mA h g−1, coulombic efficiency approaching 100% and decent cycling efficiencies over 100 cycles. A full-liquid flow-through mode is able to be realized with a controlled depth of charge. Moreover, a high energy density can be expected with highly concentrated electrolytes, guaranteeing a promising sustainable energy storage technology candidate for both stationary and mobile applications.


 Please wait while we load your content...
Please wait while we load your content...