Complete dehydrogenation of N2H4BH3 with NiM-Cr2O3 (M = Pt, Rh, and Ir) hybrid nanoparticles†
Abstract
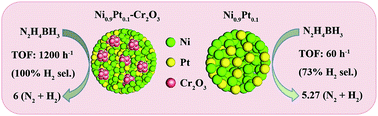
Cr2O3 doped NiM (M = Pt, Rh, and Ir) hybrid nanoparticles are successfully fabricated via a co-reduction route at room temperature. It is found that the addition of Cr2O3 not only reduces the size of the bimetallic nanoparticles and significantly increases the BET surface area as compared to that of pure Ni0.9Pt0.1 nanoparticles, but also increases the electron density of NiPt, resulting in significantly enhanced catalytic activity. The Ni0.9Pt0.1-Cr2O3 hybrid nanoparticles exhibit high catalytic activity for the dehydrogenation of hydrazine borane with a turnover frequency value of 1200 h−1 at 50 °C, which is 20 times higher than that of pure Ni0.9Pt0.1 nanoparticles and even surpasses those of most of the reported heterogeneous catalysts. The catalytic performance and H2 selectivity of NiM (M = Pt, Rh, Ir) nanoparticles are also significantly improved by the dopant Cr2O3. Other metal oxides MOx (M = Mo, W and Mn) enhance the activity of the NiPt nanoparticles as well. Our study provides a general approach for the preparation of NiM/metal-oxide hybrid nanoparticles as a type of highly efficient catalyst for the complete dehydrogenation of hydrazine borane, ammonia borane and hydrazine.


 Please wait while we load your content...
Please wait while we load your content...