Cation exchange reaction derived amorphous bimetal hydroxides as advanced battery materials for hybrid supercapacitors†
Abstract
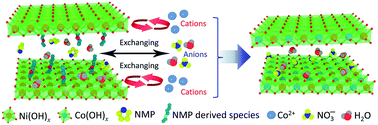
With more grain boundaries and ion diffusion channels, amorphous transition metal hydroxides are believed to exhibit better electrochemical performance than their crystalline counterparts. Currently, it is still challenging to fabricate amorphous hydroxides, especially those with multiple transition metals. In this work, a cation exchange reaction is utilized to prepare amorphous bimetal hydroxides for the first time. A cation exchange reaction can be conducted to exchange amorphous Ni(OH)2 with Co2+, Mn2+, Cu2+ and Zn2+ to obtain a series of bimetal samples and is particularly robust for the synthesis of amorphous bimetal Ni–Co and Ni–Mn hydroxides. These bimetal samples demonstrate excellent performance for charge storage, and in particular, amorphous Ni–Co hydroxide (NiCo–OH) delivers the best performance. The amorphous structure endows NiCo–OH with very high electrochemical activity, which demonstrates a specific capacity close to the theoretical one. In addition, it is proven that the Co composition plays a key role in achieving superior specific capacity, rate performance and cycling stability of NiCo–OH compared to that of pure Ni(OH)2. A hybrid supercapacitor based on the prepared amorphous NiCo–OH shows both high specific energy and power performance, further confirming the outstanding performance of amorphous NiCo–OH.


 Please wait while we load your content...
Please wait while we load your content...