A highly efficient Z-scheme B-doped g-C3N4/SnS2 photocatalyst for CO2 reduction reaction: a computational study†
Abstract
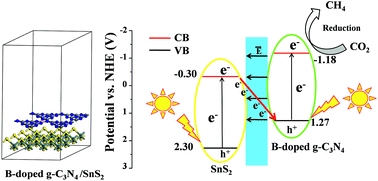
A Z-scheme heterostructure is a kind of highly efficient photocatalyst that can not only reduce the forbidden band width of the semiconductor but also promote the redox reaction in the photocatalytic system. Herein, calculations on the work function and charge density difference by density functional theory (DFT) methods prove that g-C3N4/SnS2 and B-doped g-C3N4/SnS2 are Z-scheme heterostructures. Compared to g-C3N4, the Z-scheme heterostructures show narrower band gaps, and red-shifted and stronger light absorption with remarkably improved photocatalytic activity. The reaction free energy for each step of the CO2 reduction process was calculated to further evaluate the photocatalytic activity of g-C3N4/SnS2 and B-doped g-C3N4/SnS2. The products of CO2RR catalyzed by g-C3N4/SnS2 are CH3OH and CH4, which are in good agreement with experiments, and the rate-determining step is CO2 → COOH* with a ΔG of 1.10 eV. For B-doped g-C3N4/SnS2, the optimal path is CO2 → COOH* → CO* → HCO* → CHOH* → CH* → CH2* → CH3* → CH4, and the rate-determining step is CH2* → CH3* with a ΔG of 0.40 eV. The results show that the CO2 reduction activity of B-doped g-C3N4/SnS2 is better than that of g-C3N4/SnS2. Therefore, the Z-scheme B-doped g-C3N4/SnS2 heterostructure is predicted to be a promising catalyst for CO2 reduction to CH4.


 Please wait while we load your content...
Please wait while we load your content...