Hexavalent chromium removal over magnetic carbon nanoadsorbents: synergistic effect of fluorine and nitrogen co-doping†
Abstract
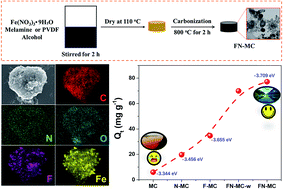
Fluorine and nitrogen co-doped magnetic carbons (FN-MCs) were obtained through a facile pyrolysis–carbonization processes. The physical and chemical properties of FN-MCs were comprehensively studied by various characterization techniques. FN-MCs were applied as adsorbents for the removal of chromium(VI) ions from aqueous solutions. The adsorption performance and kinetic characteristics were evaluated in batch mode. The results showed that the FN-MCs displayed an enhanced removal efficiency in neutral solution, higher than solo N or F doped adsorbents. The highest removal capacity in both neutral and acidic solutions was 188.7 and 740.7 mg g−1, respectively, competitive with the state-of-the-art carbon adsorbents. The unexpectedly high adsorption performance in the neutral solution can be attributed to the incorporation of the fluorine and nitrogen atoms simultaneously, which increased the negative charge density on the surface of the adsorbent. The removal efficiency increased along with the content of the heteroatoms and the defect value. The density functional theory (DFT) calculations demonstrated that the F and N co-doping reduced the adsorption energy between the Cr(VI) ions and adsorbent, thus boosting the adsorption efficiency in the neutral solution. Furthermore, the FN-MCs showed high stability in recycling tests for Cr(VI) removal.


 Please wait while we load your content...
Please wait while we load your content...