P3-type K0.32Fe0.35Mn0.65O2·0.39H2O: a promising cathode for Na-ion full batteries†
Abstract
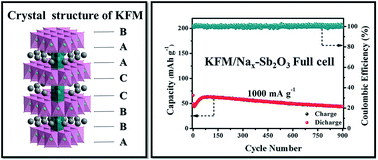
Na-ion batteries (NIBs) are very attractive candidates for large-scale electrical energy storage due to abundant Na resources, while developing air stable, low cost and toxic Co/Ni-free cathodes to achieve high energy/power density and long-life NIBs is very challenging. Herein, as a proof-of-concept experiment, rare P3-type K0.32Fe0.35Mn0.65O2·0.39H2O (KFM) made from environmentally friendly and low cost elements is first proposed and demonstrated to be a novel cathode for NIBs. Unexpectedly, the KFM exhibits outstanding electrochemical performances including high specific capacity (137.5 mA h g−1), rate capability and coulombic efficiency (ca. 100%), and long cycle stability (1000 cycles). Furthermore, a highly efficient prototype NIB was first realized using this KFM cathode and a Nax–Sb2O3 anode, which shows high energy density and power density (220.5 W h kg−1; 4340 W kg−1) even with high round-trip energy efficiency (ca. 100%) and excellent cycling stability (over 900 cycles).


 Please wait while we load your content...
Please wait while we load your content...