Stronger-than-Pt hydrogen adsorption in a Au22 nanocluster for the hydrogen evolution reaction†
Abstract
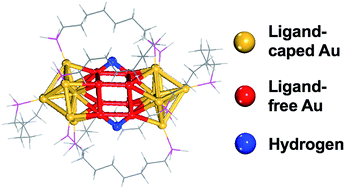
Atomically precise metal nanoclusters have recently emerged as a novel class of catalysts for the hydrogen evolution reaction. From first-principles density functional theory, we show that the eight coordinatively unsaturated (cus) Au atoms in the Au22(L8)6 cluster [L8 = 1,8-bis(diphenylphosphino) octane] can adsorb H stronger than Pt, thereby being a potentially promising catalyst for the hydrogen evolution reaction (HER). We find that up to six H atoms can adsorb onto the Au22(L8)6 cluster and they have close-to-zero Gibbs free adsorption energies (ΔGH). From the HOMO–LUMO gaps, frontier orbitals, and Bader charge analysis, we conclude that H behaves as a hydride or electron-withdrawing ligand in the Au22(L8)6 clusters, in contrast to the metallic H in thiolate-protected Au nanoclusters. Our study demonstrates that ligand-protected Au clusters with cus Au sites will be the most promising candidates for realizing Au–H nanoclusters and can act as excellent electrocatalysts for the HER.


 Please wait while we load your content...
Please wait while we load your content...