Sensing mechanism of ethanol and acetone at room temperature by SnO2 nano-columns synthesized by aerosol routes: theoretical calculations compared to experimental results†
Abstract
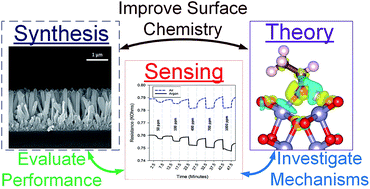
SnO2 is a semiconducting metal oxide that is broadly employed as the active sensing material in chemiresitive gas sensors. Recent studies demonstrated the capability of SnO2 sensors to detect various gases, including volatile organic compounds (VOCs), at room temperature. To explore the sensing mechanism, the adsorption of ethanol and acetone on the SnO2 (110) and (101) surface facets is investigated via density functional theory (DFT) calculations and ab initio molecular dynamics (AIMD) simulations. The role of surface oxygen defects, polarity of the VOC molecule, and pre-adsorbed oxygen species from the ambient atmosphere is explored. DFT results show that the direct adsorption of both ethanol and acetone on the (110) and the (101) surface facets is energetically favorable. The adsorption of both molecules is accompanied by the release of charge from the adsorbate gas to the surface, which promotes the sensing response. Binding strength of both molecules on the stoichiometric SnO2 (110) and (101) surfaces is greater than that on the oxygen defective surfaces. Ethanol adsorption is generally stronger than acetone due to the bipolar nature of the hydroxyl (OH) group that interacts with the surface via two distinct charge transfer modes. Minimal interaction takes place between the pre-adsorbed oxygen species (O2−) and the studied polar VOCs upon their adsorption on the reduced surfaces. Theoretical calculations are supplemented by sensing measurements for ethanol using SnO2 nanostructured thin film sensors fabricated by an aerosol chemical vapor deposition (ACVD) technique. Taken together, these results suggest that the sensing mechanism of SnO2 towards polar VOCs at room temperature can be explained by their direct adsorption on the surface rather than through their oxidation by means of ionosorbed oxygen species.


 Please wait while we load your content...
Please wait while we load your content...