What causes the anomalous aggregation in pluronic aqueous solutions?†
Abstract
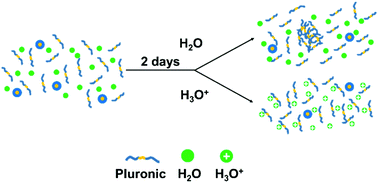
Pluronic (PL) block copolymers have been widely used as delivery carriers, molecular templates for porous media, and process additives for affecting rheological behavior. Unlike most surfactant systems, where unimer transforms into micelle with increased surfactant concentration, anomalous large PL aggregates below the critical micelle concentration (CMC) were found throughout four types of PL (F108, F127, F88 and P84). We characterized their structures using dynamic light scattering and small-angle X-ray/neutron scattering. Molecular dynamics simulations suggest that the PPO segments, though weakly hydrophobic interaction (insufficient to form micelles), promote the formation of large aggregates. Addition of acid or base (e.g. citric acid, acetic acid, HCl and NaOH) in F108 solution significantly suppresses the aggregate formation for up to 20 days due to the repulsion force from the attached H3O+ molecules on the EO segment in both PEO and PL and the reduction of CMC through the salting out effect, respectively.


 Please wait while we load your content...
Please wait while we load your content...