The role of anions in adsorbate-induced anchoring transitions of liquid crystals on surfaces with discrete cation binding sites†
Abstract
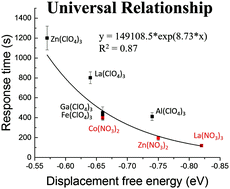
We report a combined theoretical and experimental effort to elucidate systematically for the first time the influence of anions of transition metal salt-decorated surfaces on the orientations of supported films of nematic liquid crystals (LCs) and adsorbate-induced orientational transitions of these LC films. Guided by computational chemistry predictions, we find that nitrate anions weaken the binding of 4′-n-pentyl-4-biphenylcarbonitrile (5CB) to transition metal cations, as compared to perchlorate salts, although binding is still sufficiently strong to induce homeotropic (perpendicular) orientations of 5CB. In addition, we find the orientations of the LC to be correlated across all metal cations investigated by a molecular anchoring energy density that is calculated as the product of the single-site binding energy and metal cation binding site density on the surface. The weaker single-site binding energy caused by nitrate also facilitates competitive binding of adsorbates to the metal cations, leading to more facile orientational transitions induced by adsorbates. Finally, our analysis suggests that nitrate anions recruit water via hydrogen bonding to the metal binding sites, modulating further the relative net binding energies of 5CB and adsorbates to surfaces decorated with metal nitrates. After accounting for the presence of water, we find a universal exponential relationship between the calculated displacement free energies and measured dynamic response of LCs to adsorbates for all metal salts studied, independent of the metal salt anion.


 Please wait while we load your content...
Please wait while we load your content...