Hydrogen-bonded mesomorphic complexes combining hydrophilic and fluorophilic molecular segments†
Abstract
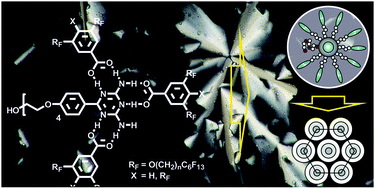
A 2,4-diamino-6-phenyl-1,3,5-triazine carrying a single oligo(ethylene oxide) (EO) chain has been investigated in binary mixtures with two-chain and three-chain semiperfluorinated benzoic acids. Mixtures of the triazine with three equivalents of the complementary acids exhibit a hexagonal columnar (Colh) mesophase. Docking of three acid molecules to the diaminotriazine nucleus leads to the formation of disc-like aggregates with a central hydrogen-bonded polar core surrounded by the peripheral fluoroalkyl chains, which self-assemble in columns arranged on a hexagonal lattice. The polar EO chains at the triazine cores do not segregate into a distinct sub-space but are included into the polar cores, providing increased flexibility of the cores with respect to conformations and available hydrogen bonding sites. In equimolar (1 : 1) triazine/benzoic acid mixtures macroscopic phase separation of excess triazine from the columnar LC phases occurs. These Colh phases are formed by [1 : 3] or [2 : 4] complexes, depending on the number of fluorinated chains. However, some of the excess triazine component is accommodated within the polar column cores, contributing to space filling and providing additional hydrogen bonding along the columns. Thus the EO chain, despite of being fixed at the periphery, assemble in the core region of the aggregates, this is distinct from the self-assembly of related alkyl or semiperfluoroalkyl substituted diaminotriazines with fluorinated benzoic acids, forming discrete disc-like [1 : 3] or rod-like [1 : 1] complexes. Obviously, the flexible EO chains decrease the effect of the aggregate shape on self-assembly and lead to an increased contribution of general amphiphilicity to self-assembly.


 Please wait while we load your content...
Please wait while we load your content...