Mechanical hysteresis in actin networks†
Abstract
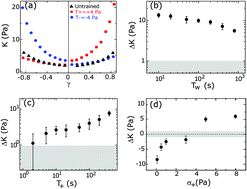
Understanding the response of complex materials to external force is central to fields ranging from materials science to biology. Here, we describe a novel type of mechanical adaptation in cross-linked networks of F-actin, a ubiquitous protein found in eukaryotic cells. We show that shear stress changes the network's nonlinear mechanical response even long after that stress is removed. The duration, magnitude and direction of forcing history all change this mechanical response. While the mechanical hysteresis is long-lived, it can be simply erased by force application in the opposite direction. We further show that the observed mechanical adaptation is consistent with stress-dependent changes in the nematic order of the constituent filaments. Thus, this mechanical hysteresis arises from the changes in non-linear response that originates from stress-induced changes to filament orientation. This demonstrates that F-actin networks can exhibit analog read–write mechanical hysteretic properties, which can be used for adaptation to mechanical stimuli.


 Please wait while we load your content...
Please wait while we load your content...