Chiral molecular face-rotating sandwich structures constructed through restricting the phenyl flipping of tetraphenylethylene†
Abstract
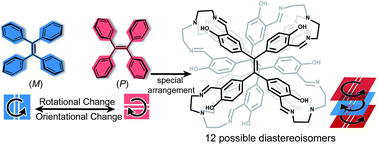
Chiral tetraphenylethylene (TPE) derivatives have great potential in chiral recognition and circularly polarized luminescence. However, they were mainly constructed through introducing chiral substituents at the periphery of the TPE moiety, which required additional chemical modifications and limited the variety of chiralities of products. Herein, we constructed a series of chiral face-rotating sandwich structures (FRSs) through restricting the phenyl flipping of TPE without introducing any chiral substituents. In FRSs, the complex arrangements of TPE motifs resulted in a variety of chiralities. We also found that non-covalent repulsive interactions in vertices caused the facial hetero-directionality of FRSs, and the hydrogen bonds between imine bonds and hydroxy groups induced excited-state intramolecular proton transfer (ESIPT) emission of FRSs. In addition, the fluorescence intensity of FRSs decreases with the addition of trifluoroacetic acid. This study provides new insights into the rational design of chiral assemblies from aggregation-induced emission (AIE) active building blocks through restriction of intramolecular rotation (RIR).
- This article is part of the themed collections: Celebrating a Century of Excellency in Chemistry at Xiamen University, 20th Anniversary of Aggregation-Induced Emission and 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...