A general electrochemical strategy for the Sandmeyer reaction†
Abstract
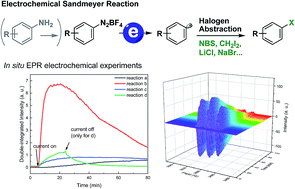
Herein we report a general electrochemical strategy for the Sandmeyer reaction. Using electricity as the driving force, this protocol employs a simple and inexpensive halogen source, such as NBS, CBrCl3, CH2I2, CCl4, LiCl and NaBr for the halogenation of aryl diazonium salts. In addition, we found that these electrochemical reactions could be performed using anilines as the starting material in a one-pot fashion. Furthermore, the practicality of this process was demonstrated in the multigram scale synthesis of aryl halides using highly inexpensive graphite as the electrode. A series of detailed mechanism studies have been performed, including radical clock and radical scavenger study, cyclic voltammetry analysis and in situ electron paramagnetic resonance (EPR) analysis.


 Please wait while we load your content...
Please wait while we load your content...