Alternating oligo(o,p-phenylenes) via ruthenium catalyzed diol–diene benzannulation: orthogonality to cross-coupling enables de novo nanographene and PAH construction†
Abstract
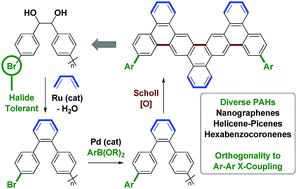
Ruthenium(0) catalyzed diol–diene benzannulation is applied to the conversion of oligo(p-phenylene vinylenes) 2a–c, 5 and 6 to alternating oligo(o,p-phenylenes) 10a–c, 11–13. Orthogonality with respect to conventional palladium catalyzed biaryl cross-coupling permits construction of p-bromo-terminated alternating oligo(o,p-phenylenes) 10b, 11–13, which can be engaged in Suzuki cross-coupling and Scholl oxidation. In this way, structurally homogeneous nanographenes 16a–f are prepared. Nanographene 16a, which incorporates 14 fused benzene rings, was characterized by single crystal X-ray diffraction. In a similar fashion, p-bromo-terminated oligo(p-phenylene ethane diol) 9, which contains a 1,3,5-trisubstituted benzene core, is converted to the soluble, structurally homogeneous hexa-peri-hexabenzocoronene 18. A benzothiophene-terminated pentamer 10c was prepared and subjected to Scholl oxidation to furnish the helical bis(benzothiophene)-fused picene derivative 14. The steady-state absorption and emission properties of nanographenes 14, 16a,b,d,e,h and 18 were characterized. These studies illustrate how orthogonality of ruthenium(0) catalyzed diol–diene benzannulation with respect to classical biaryl cross-coupling streamlines oligophenylene and nanographene construction.


 Please wait while we load your content...
Please wait while we load your content...