Chemically stable ionic viologen-organic network: an efficient scavenger of toxic oxo-anions from water†
Abstract
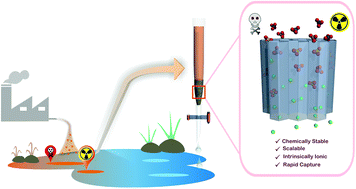
Detoxification of water has been demonstrated with a viologen-based cationic organic network (compound-1), which was stable not only in water, but also in acidic and basic media. The presence of free exchangeable Cl− ions inside the network of compound-1 and a high physiochemical stability of the materials offered a suitable scope for the capture of hazardous anionic pollutants from water. Rapid removal of the toxic water pollutant and carcinogenic chromate (CrO42−) from water was shown with compound-1. Furthermore, the oxo-anion of the radioactive isotope of technetium (99Tc), i.e. the TcO4− ion, also counts as a toxic water pollutant and by using surrogate anions (MnO4− and ReO4−), a model capture study was performed. Notably, compound-1 showed high capacity values for each of the oxo-anions and these were comparable to some of the well-performing compounds reported in the literature. Furthermore, to check the real time aspect, removal of all of the aforementioned anions from water was demonstrated, even in the presence of other concurrent anions.


 Please wait while we load your content...
Please wait while we load your content...