Kekulé diradicaloids derived from a classical N-heterocyclic carbene†
Abstract
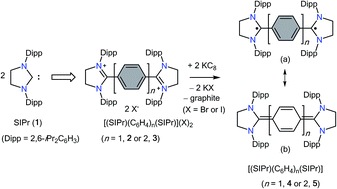
The direct double carbenylation of 1,4-diiodobenzene and 4,4′-dibromobiphenyl with a classical N-heterocyclic carbene, SIPr (1) (SIPr = :C{N(2,6-iPr2C6H3)}2CH2CH2), by means of nickel catalysis gives rise to 1,3-imidazolinium salts [(SIPr)(C6H4)(SIPr)](I)2 (2) and [(SIPr)(C6H4)2(SIPr)](Br)2 (3) as off-white solids. Two-electron reduction of 2 and 3 with KC8 cleanly yields Kekulé diradicaloid compounds [(SIPr)(C6H4)(SIPr)] (4) and [(SIPr)(C6H4)2(SIPr)] (5), respectively, as crystalline solids. Structural parameters and DFT as well as CASSCF calculations suggest the closed-shell singlet ground state for 4 and 5. Calculations reveal a very low singlet–triplet energy gap ΔES–T for 5 (10.7 kcal mol−1), while ΔES–T for 4 (29.1 kcal mol−1) is rather large.
- This article is part of the themed collection: Most popular 2018-2019 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...