Selective hydrogenation of 1,3-butadiene catalyzed by a single Pd atom anchored on graphene: the importance of dynamics†
Abstract
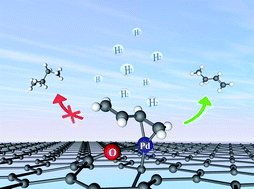
The active-site structure, reaction mechanism, and product selectivity of the industrially important selective hydrogenation of 1,3-butadiene are investigated using first principles for an emerging single-atom Pd catalyst anchored on graphene. Density functional theory calculations suggest that the mono-π-adsorbed reactant undergoes sequential hydrogenation by Pd-activated H2. Importantly, the high selectivity towards 1-butene is attributed to the post-transition-state dynamics in the second hydrogenation step, which leads exclusively to the desorption of the product. This dynamical event prevails despite the existence of energetically preferred 1-butene adsorption on Pd, which would eventually lead to complete hydrogenation to butane and be thus inconsistent with experimental observations. This insight underscores the importance of dynamics in heterogeneous catalysis, which has so far been underappreciated.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...