Green synthesis of PbCrO4 nanostructures using gum of ferula assa-foetida for enhancement of visible-light photocatalytic activity
Abstract
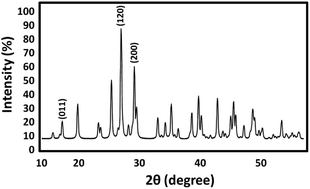
Photocatalytic selective oxidation has attracted considerable attention as an environmentally friendly strategy for organic transformations. Some methods have been reported for the photocatalytic oxidation of sulfides into sulfoxides in recent years. However, the practical application of these processes is undermined by several challenges, such as low selectivity, sluggish reaction rates, requirement of UV-light irradiation, use of additives, and instability of the photocatalyst. Pure monoclinic lead chromate nanoparticles were prepared via a new simple way as Pb and Cr sources. PbCrO4 NPs were synthesized via a green method in the presence of gum of ferula assa-foetida from Pb(NO3)2 and CrCl3 as lead and chromium resources, respectively. The structural analysis of the samples confirmed the formation of PbCrO4 nanostructures in the range of 30 ± 5 nm. The PbCrO4 nanocatalyst was thoroughly characterized by powder X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and energy dispersive X-ray spectroscopy (EDX) study. Considering the large ionic internal character and high mechanical and thermal stability as well as long-term colloidal stability, this system can be considered as a perfect nanocatalyst by using the host–guest approach. A green and ecofriendly method for oxidation of sulfides to sulfones in the presence of O2 as an oxidant was examined for the synthesised PbCrO4 NPs. The easy and applied reusability of the catalyst was observed after the completion of the reaction under visible-light irradiation.


 Please wait while we load your content...
Please wait while we load your content...