Preparation of prolinamide with adamantane for aldol reaction catalysis in brine and separation using a poly(AN-MA-β-CD) nanofibrous film via host–guest interaction†
Abstract
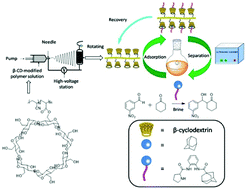
Prolinamides with double-H potential were prepared and employed as organocatalysts in asymmetric aldol reactions. The catalyst with adamantane showed improved catalytic activity, which was further enhanced by using brine as the solvent. A series of aldol reactions in brine at 0 °C provided good yields (up to 98%) with high diastereoselectivities (>99 : 1) and enantioselectivities (>99%). The prepared catalyst was adsorbed by a nanofibrous film of poly(AN-MA-β-CD) via host–guest interaction in the reaction system. The catalyst was separated from the film by applying ultrasound, with a total recovery of 96.2%. The catalyst was reused up to five times without a significant change in diastereoselectivity and enantioselectivity.
- This article is part of the themed collection: Editors’ collection: Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...