Synthesis of zeolitic material from basalt rock and its adsorption properties for carbon dioxide
Abstract
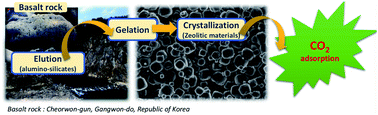
A zeolitic 4A type material was successfully prepared from natural basalt rock by applying an alkali fusion process and hydrothermal synthesis. In particular, the optimum synthetic conditions were examined at different crystallization times. Several methods such as XRD, SEM, EDX, and N2 and CO2 adsorption analysis were used to characterize the synthesized 4A type zeolite. In addition, CO2 adsorption equilibrium capacities for this basalt base zeolite were measured over temperature ranges from 283 to 303 K and pressure ranges from 0.1 to 1500 kPa in a volumetric adsorption apparatus. Then the results were compared to those of commercial zeolite. Moreover, to further investigate the surface energetic heterogeneity of the prepared zeolite, the isosteric heat of adsorption and adsorption energy distribution was determined. We found that basalt based zeolite 4A shows a CO2 adsorption equilibrium capacity of 5.9 mmol g−1 (at 293 K and 1500 kPa) which is much higher than the 3.6 mmol g−1 of the commercial zeolite as its micro-pore surface area, micro-pore volume and surface heterogeneity indicate.


 Please wait while we load your content...
Please wait while we load your content...