Copper-catalyzed oxidative coupling of quinoxalin-2(1H)-ones with alcohols: access to hydroxyalkylation of quinoxalin-2(1H)-ones†
Abstract
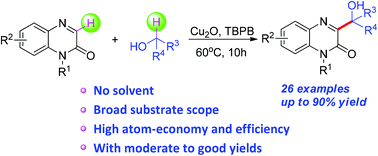
An efficient copper-catalyzed protocol for the synthesis of hydroxyl-containing quinoxalin-2(1H)-ones from the radical reaction of quinoxalin-2(1H)-ones with alcohols was developed with moderate to good yields. Quinoxalin-2(1H)-ones were coupled with alkyl radicals through direct catalytic functionalization of the α sp3 C–H bond of alcohols. The methodology demonstrates a broad substrate scope, excellent functional group tolerance, high atom economy and high efficiency, thus enabling the preparation of diverse potentially valuable hydroxyl-containing quinoxalin-2(1H)-ones.


 Please wait while we load your content...
Please wait while we load your content...