Using a traceless directing group for the silver-mediated synthesis of 3-trifluoromethylpyrazoles: a computational study on the mechanism and origins of regioselectivity†
Abstract
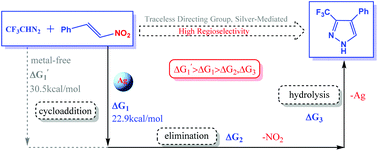
The silver-mediated one-pot synthesis of 3-trifluoromethylpyrazoles using a traceless directing group was investigated by density functional theory (DFT) calculations. In this paper, we studied computationally the two putative reaction mechanisms and origins of high regioselectivity to elucidate the principal features of this transformation. The calculated results reveal (1) the pathway involving silver-mediated cycloaddition is more kinetically favored; (2) the origin of high regioselectivity for the reaction; (3) silver(I) and nitro group can effectively improve the reaction activity and reduce the overall barrier of the reaction to 22.9 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...