Reusable Fe2O3-nanoparticle catalysed efficient and selective hydroboration of carbonyl compounds†
Abstract
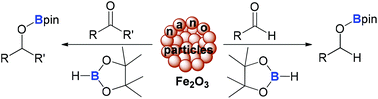
The first Fe2O3-nanoparticle catalysed hydroboration of aromatic and aliphatic aldehydes and ketones with HBpin (pin = OCMe2CMe2O) is reported. The reaction proceeds under mild conditions (room temperature) and is moderately sensitive to air. This process is applicable to a broad range of substrates with high functional group compatibility. Moreover, aldehydes are selectively hydroborated over other reducible functional groups, such as ketone, nitrile, hydroxide, alkene, amide, ester, nitro and halide groups.


 Please wait while we load your content...
Please wait while we load your content...