Enantioselective isoquinuclidine synthesis via sequential Diels–Alder/visible-light photoredox C–C bond cleavage: a formal synthesis of the indole alkaloid catharanthine†
Abstract
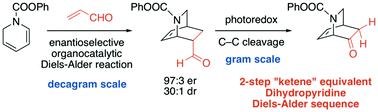
An enantioselective route to substituted chiral isoquinuclidines, present in alkaloids such as catharanthine, deserpidine, ibogamine and ibogaine, has been developed using a “ketene equivalent” approach. Organocatalyzed Diels–Alder reaction of an N-protected dihydropyridine with acrolein using a valine derived 1,2-aminoalcohol catalyst occurs with high er and dr. Subsequent Ru(Bipy)3Cl2·(H2O)6 catalyzed photoredox cleavage generates an N-protected isoquinuclidinone, providing rapid access to the core structures of a variety of important indole alkaloids. Optimized conditions for the enamine mediated photocleavage employed the use of silica gel, acetic acid (1.5 equiv.) and piperidine (3.0 equiv.), a blue LED light source, and pure oxygen rather than air. The two-step sequence utilizes acrolein as a ketene dienophile equivalent providing a new approach to asymmetric “ketene” Diels–Alder reactions. Further elaboration of the resultant isoquinuclidines permitted an enantioselective route to intermediates previously employed in total syntheses of catharanthine and deserpidine.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...