Palladium-catalyzed asymmetric annulation between aryl iodides and racemic epoxides using a chiral norbornene cocatalyst†
Abstract
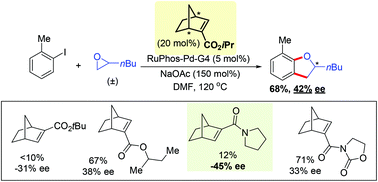
Herein, we describe our initial development of an asymmetric Pd-catalyzed annulation between aryl iodides and racemic epoxides for synthesis of 2,3-dihydrobenzofurans using a chiral norbornene cocatalyst. A series of enantiopure ester-, amide- and imide-substituted norbornenes have been prepared with a reliable synthetic route. Promising enantioselectivity (42–45% ee) has been observed using the isopropyl ester-substituted norbornene (N1*) and the amide-substituted norbornene (N7*).
- This article is part of the themed collection: Celebrating the 90th birthday of Professor Lu Xiyan


 Please wait while we load your content...
Please wait while we load your content...