Tandem dihetero-Diels–Alder and Huisgen cycloaddition reactions. Synthesis, crystal structure and hydrolysis of the novel cage phosphoranes†
Abstract
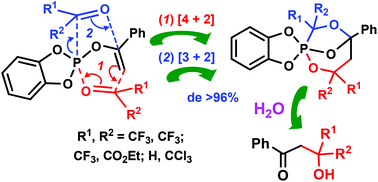
The reaction of 2-(1-phenylvinyloxy)benzo-1,3,2-dioxaphosphole with hexafluoroacetone, ethyltrifluoropyruvate and chloral leads to the formation of cage phosphoranes possessing the 1-phospha-2,6,8-trioxabicyclo[3.2.1]octane framework whose structure was established by the XRD method and NMR spectroscopy. The process involves dihetero-Diels–Alder and Huisgen 1,3-dipolar cycloaddition reactions and is accompanied by the simultaneous formation of the P–C and C–C bonds. Despite the generation of three chiral carbon atoms, the stereoselectivity of the process exceeds 96%. Hydrolysis leads to the formation of functionalized aldols and phosphonates.


 Please wait while we load your content...
Please wait while we load your content...