Dual photoredox and nickel-catalyzed desymmetric C–O coupling reactions: visible light-mediated enantioselective synthesis of 1,4-benzodioxanes†
Abstract
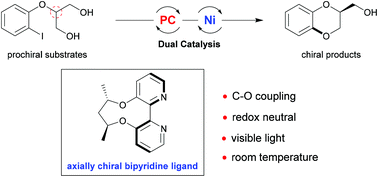
1,4-Benzodioxane widely exists as the core structure in many therapeutic agents and bioactive natural compounds. Hence, to access this kind of molecule, an enantioselective desymmetric C–O cross coupling reaction has been developed through dual visible light photoredox and nickel catalysis. Notably, the use of an axially chiral 2,2′-bipyridine ligand is the key to success. A series of chiral 1,4-benzodioxanes were afforded in high yields and moderate to good enantioselectivity under mild reaction conditions.
- This article is part of the themed collection: Celebrating the 90th birthday of Professor Lu Xiyan


 Please wait while we load your content...
Please wait while we load your content...