Photoinduced fragmentation-rearrangement sequence of cycloketoxime esters†
Abstract
A novel rearrangement of cycloketoxime esters to cyano-containing benzoates beyond the traditional Beckmann and Neber rearrangement process has been established in the presence of photocatalysis catalysis. This SET-induced structural reorganization of cycloketoxime esters is perfectly an atom economic process and distinguished by mild and safe reaction conditions that avoid acid, base and toxic cyanide salts.
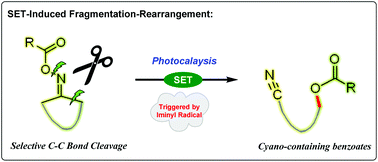
- This article is part of the themed collections: Celebrating the 90th birthday of Professor Lu Xiyan and Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...