Optically active N-alkyl aziridines via stereospecific reductive cyclization of α-mesylated acetamides†
Abstract
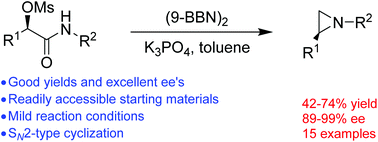
An efficient method for the synthesis of optically active N-alkyl aziridines has been realized for the first time by stereospecific reductive cyclization of optically active α-mesylated acetamides. A series of optically active N-alkyl aziridines are prepared in moderate to good yields and excellent ees. The employment of (9-BBN)2 as the reagent is crucial for the success of the transformation.
- This article is part of the themed collections: Celebrating 70 Years of Shanghai Institute of Organic Chemistry and Celebrating the 90th birthday of Professor Lu Xiyan


 Please wait while we load your content...
Please wait while we load your content...