Water-soluble naphthalene diimides: synthesis, optical properties, and colorimetric detection of biogenic amines†
Abstract
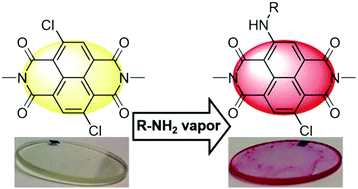
A large number of water-soluble naphthalene diimides (NDIs) bearing an electron-withdrawing chloro and electron-donating amino-substituents with varied electronic character at the core were synthesized by a two-step synthetic approach comprising imidization of 2,6-dichloronaphthalene dianhydride with L-glutamic acid or 2-dimethylaminoethylamine and subsequent nucleophilic substitution of a chlorine atom with primary amines. These new donor–acceptor NDI dyes show absorption and emission maxima beyond 500 nm and fluorescence quantum yields up to 39% in aqueous phosphate buffer solution at acidic and neutral pH values. Our studies revealed a significant effect of core amino-substituents on the optical properties of these NDIs. With increasing electron-withdrawing character of the residues of amino-substituents the absorption and emission maxima are more hypsochromically shifted and the fluorescence quantum yields are markedly increased with the highest value of 39% for NDIs containing a strongly electron withdrawing trifluroethyl group in the amino-substituent. Furthermore, the high reactivity of the core-dichlorinated NDI dye could be utilized for the optical detection of primary amines and biogenic diamines putrescine and cadaverine.


 Please wait while we load your content...
Please wait while we load your content...