Oxidation of active sp3 C–H bonds initiated consecutive intermolecular/intramolecular cyclization between glycine derivatives and o-vinylphenols: construction of a polycyclic benzofuroquinoline skeleton†
Abstract
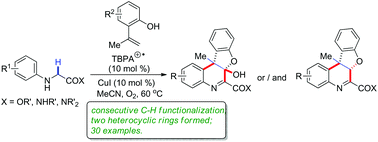
Consecutive intermolecular/intramolecular cyclization between N-arylglycine derivatives and 2-hydroxylstyrenes was realized through radical cation salt-initiated sp3 C–H bond oxidation. This reaction provided a new route to forge this biologically important polycyclic benzofuroquinoline skeleton in a single synthetic step with good functional group tolerance. The mechanistic study revealed that the oxidation of the active sp3 C–H bond initiated further functionalization of the inert C–H bond and tandem cyclization.


 Please wait while we load your content...
Please wait while we load your content...