Unsymmetrical CNN-palladacycles with geometry-constrained iminopyridyl ligands: an efficient precatalyst in Suzuki coupling for accessing 1,1-diarylalkanes from secondary benzylic bromides†
Abstract
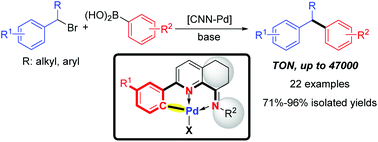
We developed a series of unsymmetrical CNN-palladacycles with geometry-constrained iminopyridyl ligands, which were conveniently prepared through a simple one-pot procedure from the corresponding 2-aryl-5,6,7-trihydroquinolin-8-ones. The palladium complexes were fully characterized by 1H and 13C NMR spectroscopy and ESI-mass spectrometry. X-ray analyses of Pd1 to Pd4 have shown their solid structures. Using these CNN-palladacycles as the precatalyst, various secondary benzylic bromides have been successfully coupled with arylboronic acids, affording 1,1-diarylalkanes with high selectivity. A TON of up to 47 000 was achieved. The scale-up reaction further demonstrated the practicality and efficiency of the developed strategy.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...