Searching for original natural products by molecular networking: detection, isolation and total synthesis of chloroaustralasines†
Abstract
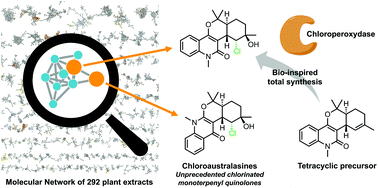
With the aim of isolating structurally original natural products, a molecular networking (MN)-based prioritisation approach has been developed and applied to a collection of 292 plant extracts. It led to the selection of a sample-specific cluster of ions detected in the bark extract of Codiaeum peltatum. The MN-guided purification of the targeted compounds afforded four unprecedented chlorinated monoterpenyl quinolones named chloroaustralasines A–C and isochloroaustralasine A. Faced with inconsistent spectral data of some previously reported quinolones, the total synthesis of the corresponding dihydroxy and chlorohydrin compounds was undertaken. The desired products were obtained in three steps, allowing the structural reassignment of two erioaustralasines. The chloroperoxidase-mediated hydroxychlorination reaction developed for the synthesis of the chlorinated quinolone showed that such complex molecules could be good substrates for this enzyme and, at the same time, raised the question of the biosynthetic origin of the non-artefactual chlorohydrin moiety.


 Please wait while we load your content...
Please wait while we load your content...