Cobalt-catalyzed intermolecular hydroacylation of aldehydes: initiation of hydride transfer enables turnover†
Abstract
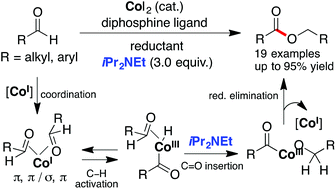
Intermolecular hydroacylation of aldehydes catalyzed by an in situ generated low-valent cobalt system was developed. Mechanistic studies disclosed that the desired hydroacylation was impeded by poor hydride donation by the respective Co(III)–H phosphine complex formed by the oxidative addition of Co(I) to the formyl C–H bond. Surprisingly, the reaction rate was significantly promoted in the presence of Lewis base N,N-diisopropylethylamine (iPr2NEt) functioning as an initiator of hydride transfer, thereby offering an excellent reaction efficiency with high substrate tolerance.


 Please wait while we load your content...
Please wait while we load your content...