The stabilizing effect of the transient imine directing group in the Pd(ii)-catalyzed C(sp3)–H arylation of free primary amines†
Abstract
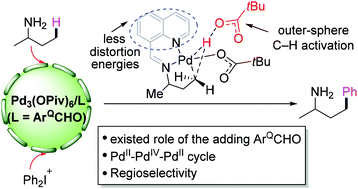
A transient directing group (DG) has been successfully applied to assist the activation of C–H bonds. In this paper, we have performed density functional theory (DFT) calculations to investigate the mechanism of ligand-assisted Pd(II)-catalyzed C(sp3)–H arylation. In the presence of a quinoline-8-carbaldehyde (ArQCHO) ligand, the reaction starts with the nucleophilic addition of 2-butylamine with the ligand to generate the transient imine DG, which binds to the Pd(II) center via bidentate coordination. The sequential C(sp3)–H activation, oxidative addition of [Ph2I]+ with a palladacycle, and C–C reductive elimination yield the final product of arylation. Instead of the traditional inner-sphere concerted metalation deprotonation (CMD) mechanism, a novel deprotonation mechanism for the rate-determining C(sp3)–H activation is discovered; that is, the methyl group is deprotonated by an outer-sphere pivalate. A comparison with the results of the reaction without the ligand indicates that the square planar geometry formed by the transient DG with Pd(II) significantly reduces the distortion energies, which ultimately makes the C(sp3)–H activation kinetically favorable.


 Please wait while we load your content...
Please wait while we load your content...